Dr Syihabuddin mendakwa ivermectin tidak berkesan berdasarkan kajian López-Medina. Syihabuddin did not bother to read the paper and he assumed his audience wouldn’t either. Beliau menyatakan di Facebook:
“Kajian berkualiti bersekala besar terkemudian membuktikan ivermectin tidak berkesan seperti Lopez Medina…”
“RCT – ….. lopez medina… dan lain-lain. Semua ni cakap ivermectin tak berkesan.”
Berikut adalah hujah Dr Syihabuddin:
“Lopez medina – daripada 476 subjek kajian, ivermectin didapati tidak berkesan.”
“Lopez Medina low risk of bias pada Izcovich, ivm not effective, n 400.”
Panjang benar Dr Syihabuddin mengulas berkenaan kajian López-Medina kenapa kononnya ivermectin tidak berkesan. Jelas beliau tidak baca sepenuhnya kertas kajian tersebut.
This is an abstract of the López-Medina randomized clinical trial (RCT):
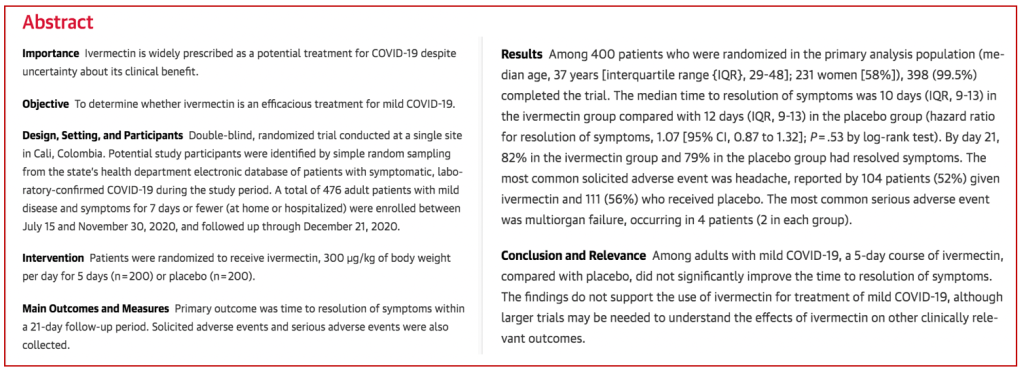
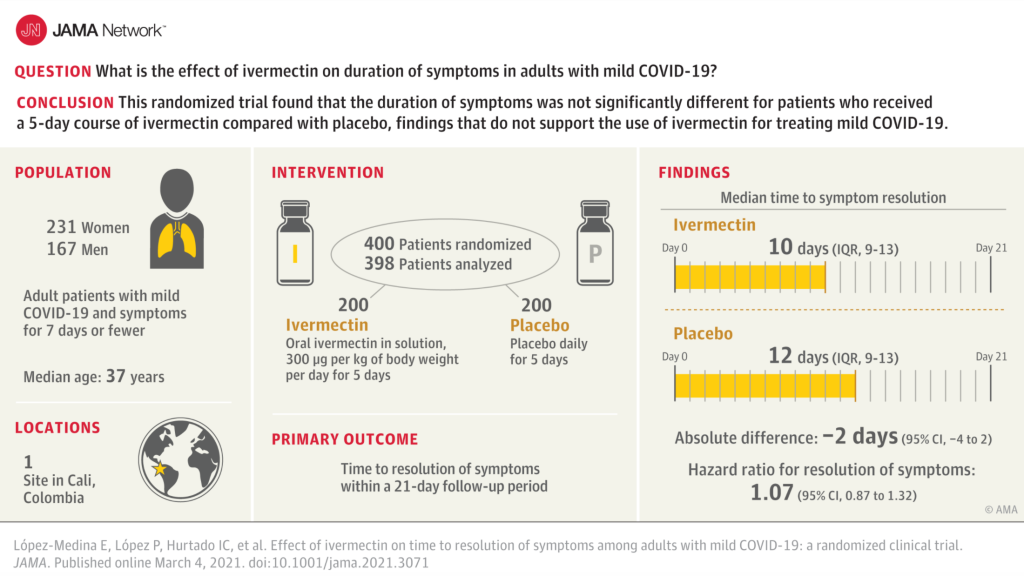
Bermula dengan 476 pesakit, 76 pesakit kemudian dikeluarkan dari kumpulan placebo kerana dengan “tidak sengaja” diberi ivermectin — kononnya silap melabelkan botol. Ini yang Syihabuddin kata “kajian berkualiti”. It was neither properly designed nor the execution properly controlled. Anda boleh menilai sendiri nanti samada the study was designed to fail.
“To me this is an error that should have stopped the study. It is my belief, and I suspect that of many others, that the editors erred allowing publication of this article.”
H. Robert Silverstein, MD, Preventive Medicine Center, Hartford
“The Lopez-Medina study in Colombia is also often cited as demonstrating that Ivermectin is ineffective. Yet it was so fraught with protocol violations that I would not have submitted the article for publication if I were the principal investigator.”
Ron Clutz
A group of 140 American doctors criticised the study through an open letter to the Journal of the American Medical Association (JAMA):
Open Letter by U.S. Doctors: JAMA Ivermectin Study Is Fatally Flawed
We the undersigned physicians present this letter to call attention to multiple, integral flaws in the Journal of the American Medical Association’s recently published paper “Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19”[1]. Above all, the data reported do not support the authors’ conclusion that ivermectin is ineffective as a treatment for mild COVID-19.
The study’s flaws span subject population, design, execution and controls. The small sample size (n = 400) had a median age of 37 and a BMI of 26, making them extremely low risk for COVID-19 hard endpoints. Faced with this low-relevance study group, the study authors improperly changed primary endpoint midway, moving the main endpoint to full symptom resolution by day 21. This self-reported subjective endpoint, obtained through telephone survey, is not credible for avoiding nondifferential nullward bias of the results.
We note other major errors in study conduct. The authors incorrectly administered ivermectin on an empty stomach, reducing drug bioavailability in lung tissue, the critical drug target, by a factor of roughly 2.5. Additionally, ivermectin is readily available over-the-counter in Colombia, where sales have been ubiquitous (1.6 doses per COVID-19 case) in Cali during the study period.[2] Lack of serum testing in the study prevented identification of subjects who may have used the drug at intervals longer than the study lookback.
Contrary to the authors’ stated conclusion, the findings suggest lower rates of disease progression, hospitalization, ICU admission, and mortality with ivermectin, which might have been confirmed to be statistically significant with a larger sample and more rigorous study design.
Indeed, notwithstanding these limitations, the available data indicate a 9% faster daily symptom resolution, which when generalized to large populations would have a meaningful impact.[3]
The severe weaknesses of this study make it uninformative about whether ivermectin is beneficial in early COVID-19 treatment. Yet, the authors misleadingly conclude that their findings “do not support the use of ivermectin for treatment of mild COVID-19”, as if they had enough quality data to make that case, which they do not.
Physicians have a solemn duty to care for patients, and randomized controlled trials can provide valuable information. But, as is the case here, they can also be flawed and must therefore always be weighed within the totality of evidence in order to provide the most appropriate patient care.
Over the past year, many government agencies, academic journals, the broader media, and medical associations have departed from historic norms and elevated the status of randomized controlled trials. Such trials are seemingly presented as the only valid basis for making clinical recommendations about COVID-19 treatment, no matter how flawed. This trend has severely hindered the ability of physicians to use clinical experience and observational trials to offer their patients guidance on early treatment for this still not well-understood infection.
We oppose this fixation on randomized controlled trials at the expense of other clinical and scientific evidence and urge medical policymakers to restore balance to the practice of medicine.
Physicians must be free to use all appropriate methodologies in determining the best approach to COVID-19, and to other conditions underlying health disparities throughout our country.
Respectfully,
Reem Abu-Sbaih DO, Greenvale, NY
Charles C. Adams MD, Ringgold, GA
Susan Allen MD, Appleton, WI and 137 other doctors
“Their approach is fundamentally flawed. It is clear that all parameters were trending to favor ivermectin at the time they truncated their study. It is of concern that this issue was not highlighted by the reviewers”
Binh Ngo, MD, Keck USC School of Medicine
More criticism from FLCCC physicians and/or their associates (source: c19ivermectin.com):
This is a phone survey based RCT with low risk patients, 200 ivermectin and 198 control, showing lower mortality, lower disease progression, lower treatment escalation, and faster resolution of symptoms with treatment, without reaching statistical significance. Authors find the results of this trial alone do not support the use of ivermectin. However the effects are all positive, especially for serious outcomes which are unable to reach statistical significance with the very small number of events in the low risk population.
RCTs have a fundamental bias against finding an effect for interventions that are widely available — patients that believe they need treatment are more likely to decline participation and take the intervention [Yeh], i.e., RCTs are more likely to enroll low-risk participants that do not need treatment to recover (this does not apply to the typical pharmaceutical trial of a new drug that is otherwise unavailable). This trial was run in a community where ivermectin was available OTC and very widely known and used.
With the low risk patient population, there is little room for improvement with an effective treatment – 59/57% (IVM/control) recovered within the first 2 days to either “no symptoms” or “not hospitalized and no limitation of activities”; 73/69% within 5 days. Less than 3% of all patients ever deteriorated.
The primary outcome was changed mid-trial, it was originally clinical deterioration, which is more meaningful, and shows greater benefit. The new outcome of resolution of symptoms includes “not hospitalized and no limitation of activities” as a negative outcome and is not very meaningful in terms of assessing how much treatment reduces serious outcomes. Using this measure could completely invalidate results – for example a treatment that eliminates all COVID-19 symptoms but has a temporary minor adverse event could be seen as worse.
Authors state that “preliminary reports of other randomized trials of ivermectin as treatment for COVID-19 with positive results have not yet been published in peer-reviewed journals”, however there were 8 peer-reviewed RCTs with positive effects published prior to this paper(and 19 total peer-reviewed studies with positive effects).
Authors advised taking ivermectin on an empty stomach, reducing lung tissue concentration by ~2.5x [Guzzo].
76 patients were excluded due to control patients receiving ivermectin. However, there was a similar percentage of adverse events like diarrhea, nausea, and abdominal pain in both treatment and control groups. These are potential non-serious side effects of treatment and suggest that it is possible that many more control patients received some kind of treatment.
Ivermectin was widely used in the population and available OTC at the time of the study. The study protocol only excluded patients with previous ivermectin use within 5 days, however other trials often monitor effects 10+ days after the last dose [osf.io].
This study reportedly has an ethical issue whereby participants were told the study drug was “D11AX22” [trialsitenews.com]. The editor-in-chief of JAMA initially offered to help with this issue, but later indicated that “JAMA does not review consent forms”, however the lead author reportedly confirmed the issue [francesoir.fr, trialsitenews.com (B), trialsitenews.com (C)].
The study protocol specifically allows “the use of other treatments outside of clinical trials”. The paper provides no information on what other treatments were used, but other treatments were commonly used at the time. Additionally, the control group did about 5x better than anticipated for deterioration, also suggesting that the control patients used some kind of treatment. Patients that enroll in such a study may be more likely to learn about and use other treatments, especially since they do not know if they are receiving the study medication.
The study protocol was amended 4 times. Amendments 2-4 are provided but amendment 1 is missing. Amendment 2 increased the inclusion criteria to within 7 days of onset, including more later stage patients and reducing the expected effectiveness. The trial protocol lists “the duration of supplemental oxygen” as an outcome but the results for this outcome are missing.
Grants and/or personal fees, including in some cases during the conduct of the study, were provided by Sanofi Pasteur, GlaxoSmithKline, Janssen, Merck, and Gilead. For more details see [trialsitenews.com (D)].
For other confounding issues see [osf.io (B)] and additional issues can be found in the comments of the article [jamanetwork.com].
Most data was collected via surveys, without physical examination. 87% medication adherence.
Objektif kajian tersebut adalah untuk menentukan samada ivermectin berkesan berdasarkan masa yang diambil untuk pesakit covid sembuh (tiada lagi symptom). The outcome was summarised as follows in the paper:

Bermaksud ivermectin memang berkesan (i) mempercepatkan kesembuhan dan (ii) lebih ramai pesakit kumpulan ivermectin sembuh dalam tempoh 21 hari berbanding kumpulan placebo .
“The 95% confidence intervals of the symptomatic parameters reported all tend to suggest ivermectin superiority over placebo in the study.”
Adesuyi Ajayi, MD, PhD, Adjunct Prof Clinical Pharmacology & Medicine, Baylor College of Medicine
Namun López-Medina menganggap perbezaan-perbezaan tersebut (yang memihak kepada ivermectin) tidak signifikan. Ivermectin “did not signficantly improve” the time to resolution of symptoms but improve it certainly did.
López-Medina tidak jujur membuat kesimpulan yang mengelirukan kononnya “the findings do not support the use of ivermectin for treatment of mild COVID-19…” Data cakap lain, López-Medina cakap lain. Lagipun López-Medina tidak menilai hasil kajian secara kesuluruhan bersama dengan bukti dari sumber-sumber yang lain iaitu terdapat kajian-kajian lain yang menunjukkan ivermectin berkesan untuk mencegah dan merawat covid (totality of evidence). Lebih wajar sekiranya López-Medina membuat kesimpulan dapatan adalah konsisten dengan dapatan kajian-kajian lain bahawa ivermectin berkesan dan bukan sekadar “did not signficantly improve”. Sepertimana dinyatakan oleh pengkritik-pengkritik kertas kajian tersebut:
“Contrary to the authors’ stated conclusion, the findings suggest lower rates of disease progression, hospitalization, ICU admission, and mortality with ivermectin, which might have been confirmed to be statistically significant with a larger sample and more rigorous study design.”
“Yet, the authors misleadingly conclude that their findings ‘do not support the use of ivermectin for treatment of mild COVID-19’, as if they had enough quality data to make that case, which they do not.“
Secara ringkasnya, adalah tidak hairan walaupun ivermectin did improve the time to resolution (masa diambil untuk sembuh) namun “did not significantly improve” atas sebab-sebab tersebut:
(i) Hanya 400 orang menyertai kajian tersebut maka pasti perbezaan antara kumpulan IVM dan kumpulan placebo akan sangat rendah dan not statistically significant. Dengan bilangan pesakit yang lebih ramai mungkin perbezaan tersebut boleh jadi statistically significant.
“Authors find the results of this trial alone do not support the use of ivermectin. However the effects are all positive, especially for serious outcomes which are unable to reach statistical significance with the very small number of events in the low risk population.” (140 US doctors)
“This is a phone survey based RCT with low risk patients, 200 ivermectin and 198 control, showing lower mortality, lower disease progression, lower treatment escalation, and faster resolution of symptoms with treatment, without reaching statistical significance” (ivmmeta.com)
(ii) Ivermectin diambil oleh pesakit dalam keadaan perut kosong maka penyerapan dalam paru-paru 2.5 kali lebih rendah berbanding diambil semasa/selepas makan. Sekiranya diambil sambil makan atau secepat mungkin selepas makan, penyerapan dalam badan akan lebih tinggi dan lebih ramai pesakit kumpulan IVM akan sembuh dalam masa yang lebih singkat.
“The authors incorrectly administered ivermectin on an empty stomach, reducing drug bioavailability in lung tissue, the critical drug target, by a factor of roughly 2.5.” (140 US doctors)
“Bioavailability of ivermectin is much greater if taken with a lipid-rich meal. Why were
participants instructed to take it on an empty stomach?” – Eric Osgood, MD, St. Francis Medical Center
(iii) Umur pesakit-pesakit (median 37 tahun) bermakna mereka berisiko sangat rendah maka ramai pesakit kumpulan placebo juga akan sembuh dengan cepat. Dengan hanya jumlah 400 pesakit, bilangan pesakit yang sembuh dari kumpulan IVM dan kumpulan placebo akan sedikit sahaja berbeza i.e. “not statistically signficant (walaupun lebih ramai pesakit kumpulan ivermectin sembuh berbanding kumpulan placebo dalam tempoh yang sama).
“With the low risk patient population, there is little room for improvement with an effective treatment – 59 [patients] /57% (IVM/control) recovered within the first 2 days to either “no symptoms” or “not hospitalized and no limitation of activities”; 73 [patients]/69% within 5 days. Less than 3% of all patients ever deteriorated.” (ivmmeta.com)
(iv) Ada kemungkinan pesakit-pesakit kumpulan placebo yang tidak mengakui mereka mengambil ivermectin. Ivermectin memang sudah digunakan khalayak ramai di bandaraya tersebut (Cali, Colombia).
“This trial was run in a community where ivermectin was available OTC and very widely known and used” (ivmmeta.com)
(v) Ada kemungkinan pesakit-pesakit kumpulan placebo yang mendapat sedikit sebanyak manfaat daripada ivermectin kerana yang dikecualikan dari kumpulan placebo adalah mereka yang telah mengambil ivermectin dalam hanya tempoh 5 hari sebelum kajian. Seeloknya tempoh tersebut adalah lebih lama (sekurang-kurangnya 10 hari) untuk lebih yakin tiada lagi kesan (residual effect) ivermectin dalam badan yang akan membantu kumpulan placebo. Maka ini menjadi satu persoalan:
“Having received ivermectin within the previous 5 days,” was an exclusion criterion. The drug is used prophylactically monthly or every 2 weeks. Do we know if participants used it during a window outside the 5 days but recently enough where residual levels could have effects? If it does have benefit, couldn’t this explain why deterioration was so much rarer than anticipated based on the literature?” – Eric Osgood, MD, St. Francis Medical Center
Maka ada adalah penting kita membezakan maksud statistically significant dan clinically important (atau clinically significant). Sesuatu berlaku yang tidak signifikan dari segi statistik tidak bermaksud tidak penting dari segi klinikal.
Statistically significant AND clinically important. This is where there is an important, meaningful difference between the groups and the statistics also support this. (The flip side of this is where a difference is neither clinically nor statistically significant).
Not statistically significant BUT clinically important. This is most likely to occur if your study is underpowered and you do not have a large enough sample size to detect a difference between groups. In this case you might fail to detect an important difference between groups. [Cindy Denisse Leyva De Los Rios]
Limiting interpretation of research results to p values means that researchers may either overestimate or underestimate the meaning of their results… Clinical significance is a decision based on the practical value or relevance of a particular treatment, and this may or may not involve statistical significance as an initial criterion. [Judith Fethney]
Medical based evidence
Satu lagi, Syihabuddin dan pentaksub vaksin (anti-ivermectin) terlalu obses dengan kajian RCT untuk membuat kesimpulan samada ivermectin berkesan. Syihabuddin cuba mengelirukan orangramai kononnya hanya RCT boleh dipercayai sebagai bukti keberkesanan ivermectin. As far as Syihabuddin is concerned, if it isn’t RCT then it doesn’t count. Ini adalah pemikiran yang sempit dan bodoh. Realitinya terdapat banyak lagi sumber lain selain RCT yang membuktikan ivermectin berkesan.
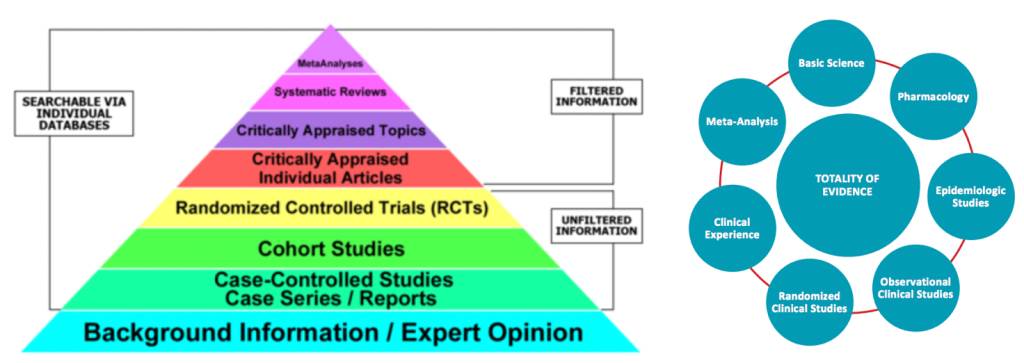
Berikut adalah kajian-kajian berkenaan ivermectin:
Bukan nak kata RCT tidak bagus tetapi RCT tidak semestinya sempurna sebagaimana didapati dengan kajian Vallejos dan López-Medini. Beberapa kritikan terhadap RCT:
“Over the past year, many government agencies, academic journals, the broader media, and medical associations have departed from historic norms and elevated the status of randomized controlled trials. Such trials are seemingly presented as the only valid basis for making clinical recommendations about COVID-19 treatment, no matter how flawed. This trend has severely hindered the ability of physicians to use clinical experience and observational trials to offer their patients guidance on early treatment for this still not well-understood infection. We oppose this fixation on randomized controlled trials at the expense of other clinical and scientific evidence and urge medical policymakers to restore balance to the practice of medicine.” — Statement of group of 140 US doctors to JAMA
“I think there are a lot of people misrepresenting the way the pyramid works. From the bottom upwards it means that there’s just more certainty. There’s no less importance given to personal opinion at the bottom of the pyramid. It’s important; it feeds up to the next level. It’s just that people right now are interested only in that one stratum and that is RCTs. We need to come with another way of medical based evidence.” — Dr Colleen Aldous, Associate Professor, Geneticist and Clinical Researcher at University of KwaZulu-Natal
“Medical care should be based on best available evidence. While randomized controlled trials (RCT) are currently considered a gold standard of study design, they are not always available and do not represent the only study format that can lead to best available evidence.” — Norbert Gleicherab, David H.Baradac
“Evidence-based practice should rely on a very broad, diverse base of evidence. RCTs would be one source, but there are lots of other sources. These sources could include Phase II trial data, epidemiological data, qualitative data and reports from the field from clinicians using an intervention” — J. Breckler, PhD, executive director of APA’s Science Directoratesay Breckler
“These critics don’t want to reject RCTs altogether. Rather, they want to supplement their findings with evidence from other methodologies, such as epidemiological studies, single-case experiments, the use of historical controls or just plain clinical experience.” — Rebecca A. Clay (American Psychological Association)
“Although RCTs are the often assigned the highest level of evidence, not all RCTs are conducted properly and the results should be carefully scrutinized. Sackett stressed the importance of estimating types of errors and the power of studies when interpreting results from RCTs. For example, a poorly conducted RCT may report a negative result due to low power when in fact a real difference exists between treatment groups” — Patricia B. Burns, MPH, Rod J. Rohrich, MD, and Kevin C. Chung, MD, MS (The Levels of Evidence and their role in Evidence-Based Medicine)
“RCTs are gold standard for detecting small (like 20%) improvements. However, RCTs are meaningless or even unethical when the treatment improves the odds by 3–6 times, as the case with Hydroxychloroquine and Ivermectin. In such situation, RCTs tend to be small or using the main ingredient incorrectly.” — Ron Clutz (How They Dissed HCQ and Ivermectin)
Saya rasa sudah cukup banyak pandangan dan kritikan dari pakar-pakar yang menunjukkan kajian RCT tidak semestinya kajian yang sempurna dan kajian López-Medina ini tidak terkecuali malah kajian tersebut jelas tidak bersekala besar dan tidak berkualiti seperti didakwa oleh Syihabuddin.
Kesimpulan
Kajian López-Medina ada beberapa masalah sebagaimana dinyatakan dan diulas di atas. Ivermectin membantu menyembuh pesakit-pesakit covid namun López-Medina membuat kesimpulan yang mengelirukan atas dasar “did not signficantly improve” atau “not statistically significant” dan ini disebabkan kajian tersebut adalah underpowered dan tidak cukup ramai pesakit-pesakit covid yang menyertai kajian untuk mengesan dengan nyata perbezaan (e.g. masa diambil untuk sembuh) di antara kumpulan IVM dan kumpulan placebo. Sungguhpun perbezaan antara dua kumpulan tersebut tidak statistically signficant namun hasil kajian yang memihak kepada ivermectin adalah clinically important atau clinically significant
Dr Syihabuddin tidak jujur mendakwa kajian López-Medina adalah “kajian berkualiti bersekala besar” dan bahawa kajian tersebut “membuktikan ivermectin tidak berkesan” yang tidak dinyatakan sedemikian oleh López-Medina. Kenyataan Syihabuddin yang mengelirukan adalah bercanggah dengan fakta-faktra yang nyata dalam kritikan-kritikan dan keterangan pakar-pakar. Dr Syihabuddin membohong.
– AA –
I love reading your blog. Your blog post is very useful and adds insight for me. Thank you for sharing.
LikeLike